
Greetings loyal readers!
I know it’s been a long time – as many of you may know, a few months ago I made the decision to move from University of Miami to the University of Colorado, and so it has been a busy, stressful, but invigorating process to reestablish myself in a new role! Unfortunately my blog activity suffered the consequences. Now, with an updated blog design, and a whole new supply of energy and enthusiasm, it’s time to pick up the pace! Lots to discuss here, but for today, we’ll do an update of the most important studies from the American Society of Clinical Oncology Annual Meeting – #ASCO 2019. This is a wordy longwinded post, and I can’t really share too many images due to copyright restrictions from the meeting… but I tried to highlight key points.
In case you need a refresher to interpret this year’s trial results: check out last year’s ASCO post for key definitions (like tumor response, progression-free survival, etc.)
Final results of ANNOUNCE: Doxorubicin plus olaratumab vs. Doxorubicin plus placebo. William Tap, MD, Memorial Sloan Kettering Cancer Center, New York, NY.
First and foremost, the final data from the Phase 3 trial of doxorubicin plus olaratumab were presented in the Plenary Session, where the top four abstracts from the meeting are revealed. As many of you may know, a press release earlier this year was released reporting that there olaratumab did not improve the outcomes over doxorubicin plus placebo, leading to the discontinuation of this drug in our treatment for patients with soft tissue sarcomas. This was a really difficult time for all of us listening, because the results from the earlier Phase 2 study that led to approval were so exciting, with patients receiving the combination with olaratumab living over a year longer than patients who only received doxorubicin. But this is why Phase 3 trials to confirm smaller studies have to happen.
Many of you have asked the important question, how did this happen and why? Dr. Jaap Verweij discussed the trial following presentation of the data, and he talked about his thoughts for how we can try to avoid trials that fall through in Phase 3.
- Nearly 30 different types of sarcomas were included in the Phase 2 and the Phase 3 trials – and with a fewer number of patients in the Phase 2, maybe those differences played a role. He argues that for future trials, running studies with individual types of sarcoma is really important to minimize the fruit basket effect – which fortunately I can show you several examples in this blog post of how this can successfully happen!
- Living longer (overall survival) is a complicated thing – because patients go on after the study to receive other treatments, like some of the new and different drugs we are using for sarcomas today. Plus, our ability to provide supportive care has gotten better – so even patients who are not getting cancer treatment may live longer.
- We need to do a better job of investigating HOW drugs work and for WHO the drugs work in the laboratory. Olaratumab blocks PDGFR activity – yet whether or not the sarcomas had PDGFR didn’t affect the ability for them to benefit from the treatment in the Phase 2. That was a red flag- we need to understand the science better and increase the chance of picking out the best drugs to try in people.
So with that – On to happier topics!
Immunotherapy updates for sarcomas
(see my post here for refresher on immunotherapy)
Doxorubicin plus pembrolizumab for sarcomas. Author: Seth Pollack, MD, Fred Hutchinson Cancer Center, Seattle, WA.
This study set out to explore combining traditional chemotherapy, doxorubicin, with the anti-PD1 immune checkpoint inhibitor pembrolizumab. Basically, checkpoint inhibitors help to re-activate tired T cells to attack cancer, but these drugs probably don’t work well if the T cells never learned to recognize the cancer cells as bad in the first place. Chemotherapy does a great job of stimulating the immune system to recognize the cancer cells, and can even create new proteins for the immune cells as the tumor cells die. So better T cell education and recognition at the beginning may provide a better army to be reactivated by the immune boosting drugs. Chemotherapy plus immune therapy has been effective in other types of cancer, leading to recent FDA approvals of the combination in breast and lung cancers.
This study included a lot of different types of sarcomas, including leiomyosarcomas, liposarcomas, pleomorphic sarcomas, chondrosarcomas, and a smorgasbord of other types. Overall it was safe, but the response rate (percent of patients whose tumors shrunk by more than 30%) was only 22% (basically one out of five patients responded). This is about the same chance of shrinkage as if you were treated with doxorubicin alone based on previous studies. However, a high percentage of patients (about 50%) had stable disease, and most of them were stable for longer than 6 months. This outcome does look a bit better than doxorubicin alone.
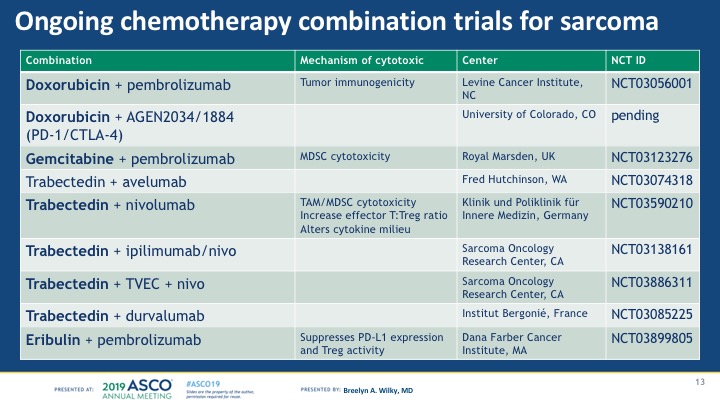
Take-home: Chemotherapy + immunotherapy is very interesting for sarcomas, but needs more study to prove that the longer disease stability suggested in this trial is real and meaningful for patients. Plus, there are quite a few trials going on combining doxorubicin or other chemotherapy drugs with immune therapy, so it’s possible a different chemotherapy drug might be a better partner than doxorubicin. See my chart below for the studies going on or about to start that combine chemo with immune drugs (as of June 1 2019).
NKTR214 plus nivolumab for patients with sarcomas. Author: Sandra D’Angelo, MD, Memorial Sloan Kettering, New York, NY.
So the next immunotherapy combo included NKTR-214 plus the anti-PD1 immune checkpoint inhibitor nivolumab. NKTR214 is a drug that boosts IL-2 function – and IL-2 is a key protein that helps to boost the initial phase of immune cell recognition and activation against the tumor cells. This study included 57 patients age 12 and up (WOW) with 10 patients with each of the following sarcomas: osteosarcoma, chondrosarcoma, leiomyosarcoma, liposarcoma, pleomorphic sarcoma, and 7 patients with vascular sarcomas (including ASPS and angiosarcomas). All patients received both drugs together.
Overall side effects were manageable and expected with immune drugs. The response rate was low, with 1 responder each with LMS, chondrosarcoma, and vascular sarcomas, and 2 responders with pleomorphic sarcoma. No responders were seen with liposarcoma or osteosarcoma. However, 40% of the liposarcoma patients, 28% of the vascular sarcomas, 20% of the leiomyosarcoma and chondrosarcomas, and 10% of the UPS had response or stable disease at 6 months.
Take-home: The combination was safe and doable, however in my opinion the responses seen were not much more than what could be expected from nivolumab alone.
Expansion of Pembrolizumab alone for patients with Pleomorphic sarcomas and dedifferentiated/pleomorphic sarcomas: (SARC028). Author: Melissa Burgess, MD, UPMC, Pittsburgh, PA.
As you may recall, the first study of immune checkpoint inhibitors in sarcoma was pembrolizumab alone. This study originally included 10 patients each with leiomyosarcomas, liposarcomas, pleomorphic sarcomas and synovial sarcoma, as well as a separate group of 40 patients with bone sarcomas. The best responses were seen in pleomorphic sarcomas, where 4 of the 10 patients had response, and 2 of the 10 patients with liposarcoma had response. The study was continued to enroll more patients with these two types of sarcomas, to determine if these impressive rates would hold up with more patients.
In this poster, Dr. Burgess showed the results of another 30 patients each with pleomorphic sarcomas and liposarcomas treated with pembrolizumab, so we had data for 40 patients with each type. Overall, 9 of 40 patients with UPS had a complete response (2 patients had disappearance of all disease) or partial response (shrinkage greater than 30%, 7 patients). So the response rate went down to 23% from the initial 40% in the first 10 patients. For liposarcoma, there were 4 patients with partial response out of 40 patients, or 10%, which went down from 20% in the first 10 patients.
Take-home: PD1 inhibitors alone are still a reasonable option for patient with pleomorphic sarcomas, with a response rate of about 23%, but 10% in liposarcomas. This data has led to most insurance companies now approving pembrolizumab alone for pleomorphic sarcomas – which is great! BUT most types of sarcomas should still be put on clinical trials with combinations instead of off label use.
Nivolumab vs. Ipilimumab/Nivolumab for patients with metastatic or unresectable GIST. Author: Arun Singh, MD, Santa Monica, CA
While most gastrointestinal stroll tumors (GIST) are treated with oral chemotherapy drugs that block KIT and PDGFR, like imatinib, sunitinib, and regorafenib, as well as new drugs avapritinib and ripretinib (will be the topic of an up-and-coming blog post), there are still very few treatment options for patients whose disease stops responding to these drugs. The immune system has been shown to be important in GIST, with the ratio between killer T cells, and suppressive T regulatory cells important for prognosis and sensitivity to imatinib. This study compared the PD-1 inhibitor nivolumab alone vs. ipilimumab/nivolumab for GIST patients. 29 patients were treated on the study so far, 16 in the nivolumab group and 13 in the ipilimumab/nivolumab group. 8 of 16 patients with GIST had stable disease on nivolumab, 5 patients for over 6 months. There was one patient on the ipilimumab/nivolumab group who had shrinkage of the tumors by over 70%. Two patients on ipilimumab/nivolumab have not had progression of the disease for over 100 weeks, both with exon 17 mutations (pretty rare)!
Take-home: Checkpoint inhibitors can be active in some patients with GIST, but we need a lot more research to understand how to find those patients, including potentially those patients with exon 17 mutations. May be worth trying in other types of GIST if no other effective treatment options, ideally on a clinical trial.
Other honorable mentions:
- Pexidartinib (targets suppressive immune cells called macrophages in tumors) plus sirolimus showed durable stable disease (median 18 weeks) in patients with malignant peripheral nerve sheath tumor (MPNST) and being expanded for more patients in Phase II trial – NCT02584647
- Apatinib plus Camrelizumab (anti-PD1 inhibitor) for 43 patients with osteosarcoma (older than 11 years). In Katie Janeway’s discussion, she showed several trials of blood vessel blocking drugs (TKIs) and the benefits being seen in osteosarcomas that are no longer responding to chemotherapy. Some of the drugs that have been previously studied include regorafenib, sorafenib, and pazopanib – all good options for these patients. Overall these benefits including responses for between 7-14% of patients, but between 30-60% of patients having stable disease at 4 months. This new study combined apatinib, a TKI, with a PD-1 inhibitor. 62% of patients had stable disease or better at 4 months, and 44% at 6 months. Tumor shrinkages were seen in 9 of 43 patients (22%). If you compare to a previous study of apatinib alone, the shrinkage rate was higher at 43% with apatinib alone, but the dose was higher in that study. Take-home: Needs more study but very interesting!
Other trials of targeted treatments and chemotherapies
It’s impossible to capture all of the remarkable research presented at ASCO – so without a doubt there is important work not included here. But the following studies I felt were the most critical to our day to day routines as sarcoma doctors, particularly since this year there were several studies that supported particular treatments for particular sarcoma subtypes. As an oncologist it’s always easier to provide information based on my patient’s specific disease, rather than data built on all-comers! The following key studies are a testament that by working together, we can do trials in rare, specific subtypes of sarcomas and get better outcomes!
Gemcitabine plus docetaxel vs. gemcitabine plus pazopanib for patients with advanced soft tissue sarcomas. Author: Neeta Somaiah, MD, MDACC, Houston, TX.
90 patients were randomized to either gemcitabine plus docetaxel (standard “gem/tax” although higher dosing of docetaxel than usual), or gemcitabine plus the TKI pill pazopanib, again at highest doses. Open to soft tissue sarcomas, NOT liposarcomas. About 30% of patients in each arm had leiomyosarcomas. Overall, the outcomes were almost identical regardless of which combination was used, for all sarcomas and the leiomyosarcomas alike. 8 of 44 patients achieved partial response with gem/tax (18%), and 5 of 43 patients in the gemcitabine/pazopanib arm (12%). Changes of having a response or stable disease as the best outcome were nearly identical in both groups at 66% and 68%. Median progression-free survival was identical (catch the trend?) of 4.1 months. About 80% of patients required dose reductions, and 93% of patients required dose skipping with gemcitabine/pazopanib, and 58% of gemcitabine/docetaxel. Serious side effects were about 20% in both treatments.
Take-home: for patients who can’t tolerate docetaxel side effects, for example in the case of allergic reactions, could consider gemcitabine/pazopanib which works about the same. Gemcitabine/pazopanib was better than historically one would expect with gemcitabine alone or pazopanib alone. Higher rates of low white blood cells and red blood cells and the risk of liver side effects were important to watch for with this combination.
Cabozantinib for GIST– Patrick Schoffski, MD, EORTC Soft Tissue and Bone Sarcoma Group.
In this study, called CABOGIST, the investigators looked at another TKI with broad impact against multiple resistance pathways in GIST (similar to regorafenib). The study included patients who had GIST that progressed on imatinib and sunitinib, but no other TKIs. The main side effects included electrolyte and liver test abnormalities in <10%. The other common side effects included diarrhea, hand-foot syndrome, weight loss, and hypertension (high blood pressure), all of which are expected with this drug. 30 of the 50 included patients were free from progression at 12 weeks. Median PFS was 6 months, with 21.9% of patients progression-free at one year. 14% had partial response, 66% achieved stable disease. Patients with exon 11, 17, and 9 mutations had responses. Take-home: these results were similar or slightly better compared to historical outcomes from the current standard of care, regorafenib, with a similar side effect profile.
Other recent studies that have really changed the treatment landscape for GIST will be expanded upon in the upcoming GIST blog post, but this other study was presented in poster form, so for a teaser:
Avapritinib (BLU-285) – the NAVIGATOR study. Michael Heinrich, MD, Oregon Health Sciences University, Portland, OR.
121 patients with KIT-mutated GIST were enrolled in the “4th line” setting, meaning the disease had grown despite imatinib, sunitinib, and regorafenib, and 43 patients were also enrolled with PDGFR exon 18 mutations in any line of treatment (most with the resistant D842V subset). 86% of patients with PDGFR exon 18 had complete or partial tumor shrinkages, really remarkable outcomes and better than any other drug for this subset to date! In the 4th line KIT-mutated GIST patients, 17% had tumor shrinkage, which lasted for a median 10.3 months, and median PFS of 3.7 months. Important to note with this drug is a very high risk of changes in mental thinking and performance (cognitive effects), which were mostly mild and reversed with drug stoppage, but seen in 41% of patients. 8% were serious. Take-home: No-brainer for patients with PDGFR exon 18 mutant GIST. For 4th line, it does have activity but need to be aware of the potential cognitive side effects.
ABI-009 (nab-sirolimus) for PEComas (perivascular epithelioid cell tumors). Andrew Wagner, MD, PhD, Dana Farber Cancer Institute, Boston, MA.
PEComas are insanely rare sarcomas that are usually driven by a genetic signaling pathway called the mTOR pathway, due to missing genes that suppress tumor growth such as TSC1 or TSC2. Nab-sirolimus (ABI-009) used in this study is a newer IV drug that blocks this pathway. This is the first clinical trial to look at any drug for PEComa. Until now, we have been using a variety of mTOR inhibitors to treat these tumors off label (IV temsirolimus, or oral everolimus). The most common side effects with ABI-009 included oral sores called mucositis, rash, and gastrointestinal side effects (nausea, vomiting, diarrhea, etc.) Partial responses (shrinkage of more than 30%) were seen in 42% of patients (13 of 31). What’s really impressive is that 62% of patients who had tumor shrinkage are still on therapy, with 3 patients on for more than 1 year and 3 patients on treatment for more than 2 years. So if it works for the patient, it really works! This trial was designed to apply for FDA approval, so hopefully we will have a specific drug for PEComas soon!
Abemaciclib for dedifferentiated liposarcoma. Mark Dickson, MD, Memorial Sloan Kettering Cancer Center, New York, NY.
This Phase 2 trial looked at abemaciclib, a drug that is related to palbociclib that blocks a protein called CDK4, which is amplified in nearly all dedifferentiated liposarcomas. These types of drugs have been used heavily in breast cancers, and so we have benefited because of small trials that showed benefit in dedifferentiated liposarcomas. Although very few patients have tumor shrinkages, about 60% can have stable disease at 3 months. The side effects of palbo are problematic because many patients have problems with blood counts, particularly because most of them will have had a lot of chemotherapies. This newer drug has less lowering of white blood cell counts although more GI side effects (nausea, vomiting, diarrhea). This study included 30 patients, and they found that 76% (22 patients) did not progress at 3 months. Median PFS was 30 weeks (over 7 months), and some patients have stayed on study for over 3 years with stable disease. While again it is bad form to compare to other studies, the median PFS for palbociclib was 18 weeks, so this drug certainly seems promising for further study.
Tazemetostat for epithelioid sarcoma. Silvia Stacchiotti, MD, Instituto Tumori, Milan, Italy.
Epithelioid sarcoma is another rare but devastating type of soft tissue sarcoma, which is aggressive and doesn’t respond particularly well to chemotherapy. About 20% of these tumors will have a loss of a master regulator gene called INI1. If INI1 is lost, there is an increase in another regulatory gene called EZH2. This new drug Tazemetostat is an inhibitor of EZH2. The study included 62 patients with epithelioid sarcomas who had lost INI1. 9 patients achieved partial response (15%) with another 56% of patients achieving stable disease. The median PFS was 23.7 weeks, with 21% free from progression at 1 year. While the data is still early, those patients that do achieve tumor shrinkage appear to hold on to that response for likely well over a year. So in response to these outcomes, the drug company has filed an application for approval with the FDA.
Well that’s the highlights, folks… let me know if you have any questions, and again thanks so much for not giving up on me! More posts to come, including spotlights on new clinical trials for synovial sarcomas, GIST, and ASPS, all of which are really having rapid changes in treatment strategies as a result of research. We are slowly moving the needle towards better outcomes for our patients!
Always read your communications and congrats on your new position. Pls keep the info coming.
Do you know if after olaratumab’s removal any new treatments are in the pipeline?
Thx
Gk
LikeLike
and you just reminded me that I forgot to include the olaratumab removal…thanks for pointing that out! time to quickly update this post! right now there’s not another big combo with doxorubicin in the pipeline, probably the most promising addition right now is the checkpoint inhibitors. we will see how that impacts our treatments but still too early to say.
LikeLike
Thank you, Dr. Wilky! Your posts (with interpretations) are so valuable to all of us! 🙂
LikeLike
Interesting reading
LikeLike
Gist Exon 9 and 17 have you any recommendations for treatment many thanx.
LikeLike
Dear all, please note that I made a mistake in reporting the incidence of cognitive effects in the avapritinib study for GIST – the rate of patients having cognitive effects was 41%, NOT 84%. Apologies for this and it has been corrected.
LikeLike
Very interesting and always uplifting to see research for sarcoma being done. I have stage 4 osteoblastic osteosarcoma with tumors everywhere. I was diagnosed with cancer in 9/15 just they didn’t know what kind until end of January 16. Biopsies of the liver(5) and sternum and finally my scalp, all were inconclusive till my hip fractured while having the tumors removed from my scalp woke up with a complete fracture and had emergency hip replacement and the orthopadiec surgeon who discovered my tumors sent sample to a pathologist he knew from John Hopkins. Its been very difficult dealing with this as I was very active runner and it has been disabling. However for the last almost 2 years I have been on Keytruda and have been holding stable. I also get Xgeva injections every 28 days. Just thought it might be something that would benefit some other people who have the same thing that I have. Have a blessed day and thank you for continuing to seek a cure.
LikeLike